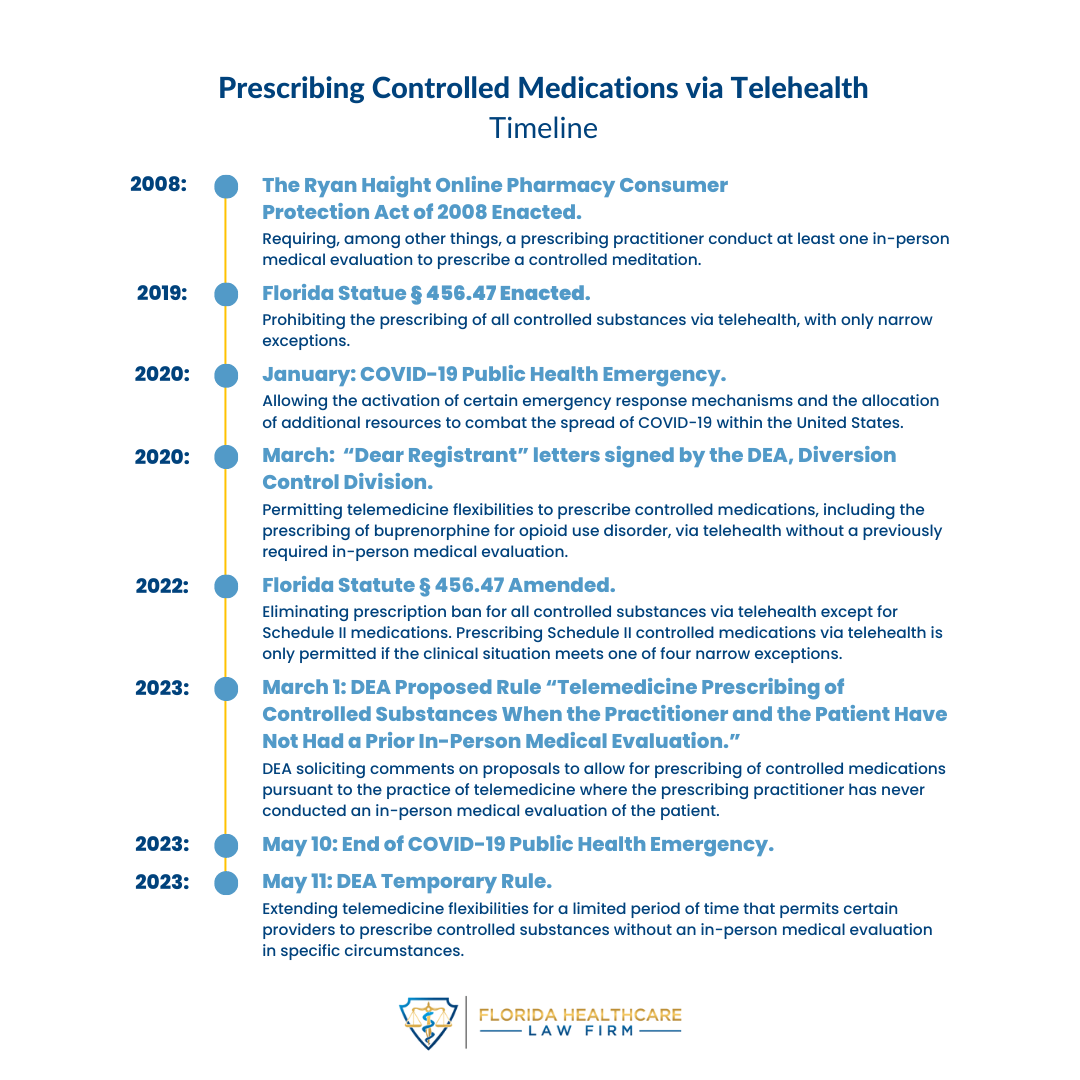
 In its latest Temporary Rule, the Drug Enforcement Administration (“DEA”) extended its rule in response to the COVID-19 Public Health Emergency (“COVID-PHE”) permitting authorized providers to prescribe controlled substances without an in-person medical evaluation in specific circumstances (referred to as, “telemedicine flexibilities”). The DEA extended the telemedicine flexibilities for six months from May 12, 2023, through November 11, 2023, subject to certain conditions. If a patient and a practitioner have established a telemedicine relationship on or before November 11, 2023, however, the same telemedicine flexibilities that governed the relationship to that point are permitted until November 11, 2024.
In its latest Temporary Rule, the Drug Enforcement Administration (“DEA”) extended its rule in response to the COVID-19 Public Health Emergency (“COVID-PHE”) permitting authorized providers to prescribe controlled substances without an in-person medical evaluation in specific circumstances (referred to as, “telemedicine flexibilities”). The DEA extended the telemedicine flexibilities for six months from May 12, 2023, through November 11, 2023, subject to certain conditions. If a patient and a practitioner have established a telemedicine relationship on or before November 11, 2023, however, the same telemedicine flexibilities that governed the relationship to that point are permitted until November 11, 2024.
i. How This May Impact Your Practice, Florida Considerations, and Future Predictions
While the DEA seeks to avoid lapses in care to patients who have relied on the telemedicine flexibilities through the Temporary Rule, the DEA noted—several times—that this extension merely extends the telemedicine flexibilities for a limited period of time. Providers who prescribe controlled medications, including testosterone for hormone replacement therapies and functional medicine practices, should stay alerted and informed of the upcoming changes to their prescriptive authority via telemedicine platforms. The DEA stated that it strongly supports policies that promote access to effective and safe treatment for opioid use disorder through telemedicine platforms and ensuring continued access to necessary controlled medications past the COVID-Public Health Emergency; the same may not be true for treatments utilizing controlled medications such as hormone therapies via telehealth. One of the purposes of the extension is to allow these providers to transition from a reliance of the availability for controlled medication prescriptions, and to give providers enough time to comply with the new standards in the DEA’s final rules. Prescribing practitioners and telemedicine companies and providers should use the extension as an opportunity to transition and comply with the upcoming final rule. Providers should also be prepared to change prescribing practices to patients via telemedicine, especially for Schedule II controlled medications, where it may be impracticable to conduct an in-person evaluation. The DEA stated that it remains vigilant on prescribing practices that it deems problematic through telemedicine capabilities, and the agency noted that it is actively investigating certain telemedicine companies it believes may have engaged in problematic prescribing practices.
Looking forward, the DEA plans to issue one or more final rules that extend, and eliminate, certain telemedicine flexibilities on a permanent basis. The final rule may likely permit practitioners to prescribe controlled substances through telehealth without an in-person evaluation to treat opioid use disorder based on the DEA’s attitudes towards telemedicine flexibilities. Prescribing providers who rely on telemedicine to prescribe controlled substances, especially those not to treat opioid use disorder, should be aware of the development of the final rule(s). With the growth of telemedicine, the DEA may also likely implement at least some telemedicine flexibilities permanently, as well. It is important to remain up to date and prepared.
The Temporary Rule applies to all areas in the United States. Florida, however, has its own restrictions on prescribing controlled substances via telehealth. Although Florida generally permits the prescribing of controlled substances via telehealth, Fla. Stat. § 456.47(2)(c) limits the ability of a telehealth provider to prescribe Schedule II controlled substances to four narrow instances. Taken with the Temporary Rule extending telemedicine flexibilities without an in-person evaluation, including for Schedule II controlled substances, Florida telehealth providers still cannot prescribe Schedule II controlled substances via telehealth to Florida patients unless it is for treatment that qualify for the limited exceptions of Section 456.47(2). Providers should also be aware of rules issued by relevant state Boards surrounding the prescribing of controlled medications. Telehealth providers in Florida must therefore be aware of both Florida and federal rules and regulations to ensure that they have compliant prescribing practices.
The DEA’s changes have and will affect businesses and practices that rely on telemedicine for treatment and may require substantive changes to telemedicine prescribing practices to be compliant with the changing rules. This temporary rule is only one of many expected rules that are evolving the landscape of telemedicine in the post-COVID world.
