Healthcare reform alone is enough of a Rubik’s Cube, but CMS and the OIG has been especially well-staffed these days, enough so that their offices are turning out new laws and interpretations at an alarming rate. Though it may seem overwhelming, physicians need to work harder than ever to stay on top of the changes.
Health Information Technology (HIT)
The physician incentive payments/penalty provisions that piggybacked their way onto the federal healthcare reform law have physicians concerned and scrambling. IT vendors and advisors are drawn to the opportunities the new law has created; and physicians need to be educated and wise.
The so called “HITECH” provisions of the federal healthcare reform law create a pot of about $34 Billion worth of incentive payments for eligible professionals and hospitals that attain meaningful use of certified electronic healthcare records (EHR) technology. To obtain any money, eligible parties will have to demonstrate full compliance no later than 2015, and earlier (2011!) if they want the full benefit. Medicare has allocated roughly $44K worth of incentives for each compliant physician; and Medicaid offers another $20K roughly, but the real incentive is not the money; it’s the fact that financial penalties apply if you don’t comply by 2015.
Financial incentives are available for eligible professionals who use certified HIT which satisfies the “meaningful use” regulations, which were issued August 2010. They are complex and limited by time lines which industry insiders claim to be unreachable. Vendors are, nevertheless, selling and physicians are buying software and solutions in hopes they will qualify for the incentive payments. Physicians should make sure that their contracts with such vendors protect them by requiring the solutions to be certified and meet the meaningful use guidelines.
Healthcare Reform
Though everyone is scared about how healthcare reform will unfold, remembering the past may help. The fact is the concepts in the Act are not new. For instance, IPAs, PHOs, capitation and the like are the cornerstone of the reform. Physicians have seen these before, though not on a government mandated basis. Moreover, where those models were once purely financial, there is a heavy clinical outcome component woven into the regulations.
No matter how one views it, the Act creates huge opportunities for physicians and others. Risk based compensated Accountable Care Organizations (ACOs) are slated to be the new platform for healthcare delivery. Good news for PCPs: regulators and think tankers think that physicians, especially primary care physicians, are the best positioned to lead the ACO development charge. That said, the form the ACOs will take is completely unclear and is expected to unfold over a period of ten years. Like technology vendors, physicians have to be wary of anyone who has something to sell at this time. One size does not fit all! IPAs might be a great vehicle to start. Capitated models are familiar, but a bundled payment methodology may work better in some circumstances. One thing that is certain: whatever business model a physician explores ought to be able to bear financial risk (e.g. capitation or bundled payments) and measure clinical outcomes, because both elements will form the basis of payments of the future. Though specifics about the future of healthcare are unavailable, the following is a fair list of what’s likely:
- Movement away from fee for service payment to risk based compensation;
- The prevalence of IT & EMR;
- The need to demonstrate clinical effectiveness;
- An expanded role of primary care physicians;
- Expansion of concierge type services;
- Employment of physicians by hospitals;
- The development of larger medical practices;
- More patients (through insurance mandates and expansion of Medicaid eligibility);
- Expanded use of “physician extenders” (as the PCP shortage worsens); and
- Increased enforcement in the area of healthcare fraud (civil & criminal).
OIG and CMS Pronouncements
May was a busy month for healthcare regulators. SMS issues the Ambulatory Surgery Center Waiting Area Separation Requirements, which has had the effect of preventing creative business opportunities between ACSs and other healthcare businesses.
Additionally, the OIG recently issued an Advisory Opinion which makes it very difficult for imaging centers to do prior authorizations for referring physicians.
Fraud and Abuse
If the first 2/3 of 2010 are any indication of the future in healthcare law, healthcare business professionals have a lot to keep up with. Enforcement by the Justice Department and the Office of Inspector General is in full swing. Already, for instance, nearly $2 million has been repaid as the result of employing a person who has been excluded from a federal healthcare program. Examples include:
Read On at www.FloridaHealthcareLawFirm.com

 There’s certainly a lot of enforcement activity against compounding pharmacies these days. The ramp-up began around 2012 after a
There’s certainly a lot of enforcement activity against compounding pharmacies these days. The ramp-up began around 2012 after a 
 By:
By: 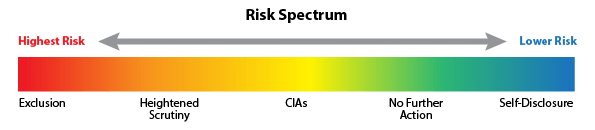

 By:
By: